
WebThe spin only magnetic moment of `[MnBr_(4)]^(2-)` is 5.9 B.M. Geometry of the complex ion is WebThe spin only magnetic moment depends on the number of unpaired electrons present in the central metal ion in the complex. If the central metal has unpaired electron, the complex.
The Spin Only Magnetic Moment Of Mnbr4, The spin only magnetic moment of `[MnBr_(4)]^(2-)` is 5.9 B.M. Geometry of the complex ion is, 4.05 MB, 02:57, 4,165, Doubtnut, 2020-05-03T07:47:26.000000Z, 19, Q. The spin only magnetic moment of [MnBr4]2- in 5.9 BM predict t, www.doubtnut.com, 800 x 94, png, , 20, the-spin-only-magnetic-moment-of-mnbr4, KAMPION
WebSpin only magnetic moment of [MnBr6]^4– is____ B.M. (round off to the closest integer) ← Prev Question Next Question →. 0 votes . 1.1k views. asked Jul 12,. WebClick here👆to get an answer to your question ️ The spin only magnetic moment of [MnBr.12 is 5.9 BM. Predict the geometry of the complex ion ? Solve Study Textbooks Guides. Join. WebBut the fact that the magnetic moment of the complex ion is 5.9 BM μ s = n (n + 2) ⇒ 5.9 = n (n + 2) ⇒ n = 5 ∴ The complex has 5 unpaired e − suggests that it should be tetrahedral. WebMagnetic moment is of two types 1) spin magnetic moment 2) orbital magnetic moment. Magnetic moments are used to obtain the information about the oxidation number and. WebThe Spin Only Magnetic Moment Of [MnBr4]2- is 5.9 B.M. Geometry Of The Complex Ion Is Home. NEET. Inorganic Chemistry . Coordination Compounds . Why Kaysons ? Video. WebThe spin-only magnetic moment of [MnBr4]2–is 5.9 BM. The geometry of the complex ion is- 1. Square planar 2. Tetrahedral 3. Octahedral 4. None of the above NCERT Solved. Web7. The final major product obtained in the following sequence of reactions is -. 8. Which of the following compounds can be detected by Molisch's test : 9. P h− C H 2 − Cl K CN (1). WebThe spin only magnetic moment of an ion is equal to n (n + 2) where n is the number of unpaired electrons in the ion. The spin only magnetic moment of S c 3 + is 1. 7 3 B. M.. WebThe oxidation state of Mn in [MnBr 4] 2− is +2 and since its magnetic moment is 5.92 BM This implies n(n+2) = 5.92 BM, n=5. Hence Mn has 5 unpaired electrons. As Br is a weak.
Latest The spin only magnetic moment of `[MnBr_(4)]^(2-)` is 5.9 B.M. Geometry of the complex ion is New
Reviews The magnetic moment of `[MnX_(4)]^(2-)` is 5.9 BM. The geometry of the complex ion is: (X=monodentat New
What to know about The Spin Only Magnetic Moment Of Mnbr4 latest
The spin only magnetic moment of `[MnBr_(4)]^(2-)` is 5.9 B.M. Geometry of the complex ion is
News Q. The spin only magnetic moment of [MnBr4]2- in 5.9 BM predict t update
![The Spin Only Magnetic Moment Of Mnbr4 Q. The spin only magnetic moment of [MnBr4]2- in 5.9 BM predict t](https://d10lpgp6xz60nq.cloudfront.net/question-thumbnail/en_571113123.png)
Articles The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM
![The Spin Only Magnetic Moment Of Mnbr4 The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM](https://i.ytimg.com/vi/no_ZGBD689M/maxresdefault.jpg)
Photos The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM more
![The Spin Only Magnetic Moment Of Mnbr4 The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM](https://learnqa.s3.ap-south-1.amazonaws.com/images/161059055311088452881610590553.png)
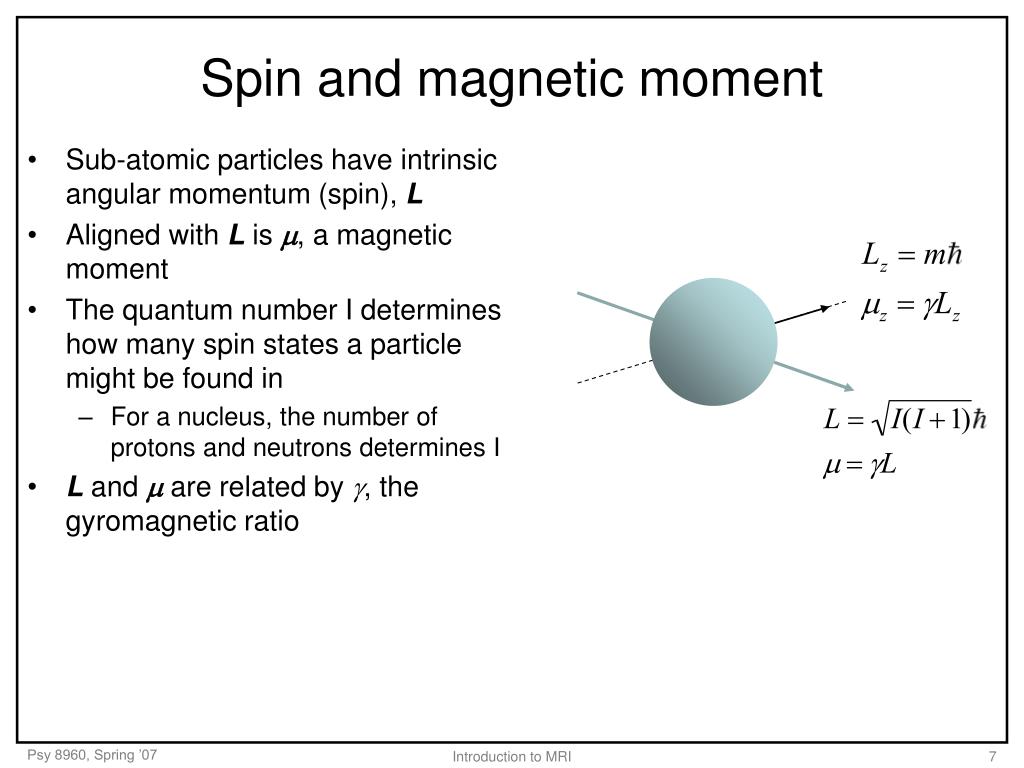
Discussion PPT - Introduction to MRI: NMR PowerPoint Presentation, free download trending
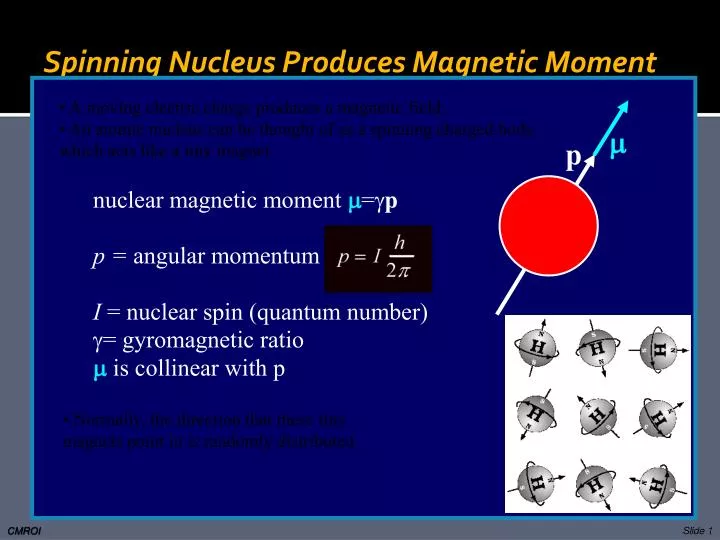
New PPT - Spinning Nucleus Produces Magnetic Moment PowerPoint Presentation trending
Here The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM
![The Spin Only Magnetic Moment Of Mnbr4 The spin only magnetic moment value of `[MnBr_(4)]^(2-)` ion is `5.9BM](https://learnqa.s3.ap-south-1.amazonaws.com/images/16105905534615382531610590553.png)
Look The spin only magnetic moment of [MnBr4]^2 - is 5.9BM . Predict the more
![The Spin Only Magnetic Moment Of Mnbr4 The spin only magnetic moment of [MnBr4]^2 - is 5.9BM . Predict the](https://dwes9vv9u0550.cloudfront.net/images/10854149/cd59c2a5-98dd-4349-b37d-c99b3ae1c987.jpg)
About The spin only magnetic moments of [Mn(CN)6]4 - & [MnBr4]2 - in bhor Latest
![The Spin Only Magnetic Moment Of Mnbr4 The spin only magnetic moments of [Mn(CN)6]4 - & [MnBr4]2 - in bhor](https://hi-static.z-dn.net/files/d8b/de7893f7e1d19b613f4a642c555218bf.png)
New Using rare earth magnets in medical devices - StarFish Medical

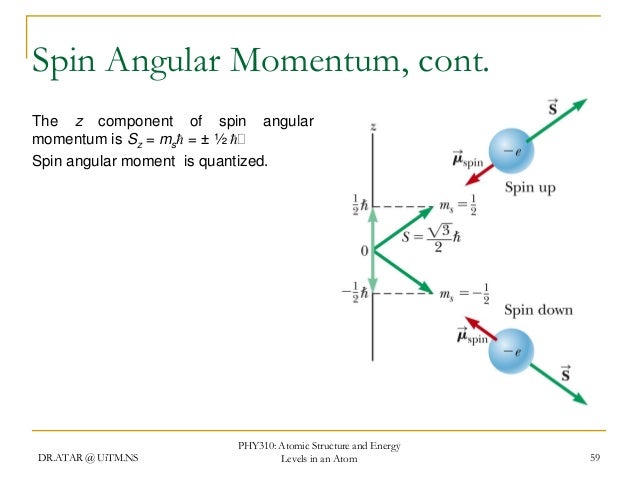
Photos Phy 310 chapter 4 Latest
![Watch video The spin only magnetic moment of `[MnBr_(4)]^(2-)` is 5.9 B.M. Geometry of the complex ion is Now The spin only magnetic moment of `[MnBr_(4)]^(2-)` is 5.9 B.M. Geometry of the complex ion is](https://img.youtube.com/vi/dWw7qgw8cX4/maxresdefault.jpg)
![Watch video The magnetic moment of `[MnX_(4)]^(2-)` is 5.9 BM. The geometry of the complex ion is: (X=monodentat Now The magnetic moment of `[MnX_(4)]^(2-)` is 5.9 BM. The geometry of the complex ion is: (X=monodentat](https://img.youtube.com/vi/gLjfefv2Wng/maxresdefault.jpg)
إرسال تعليق